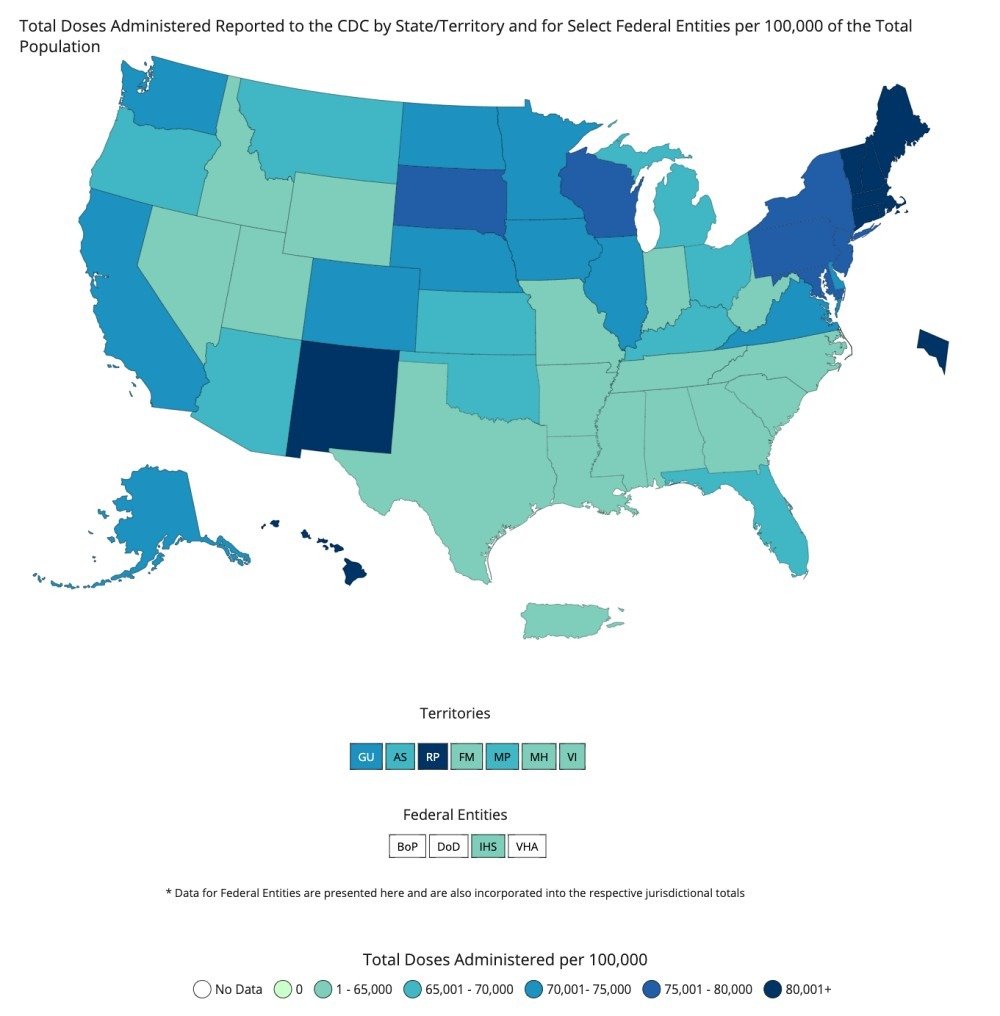
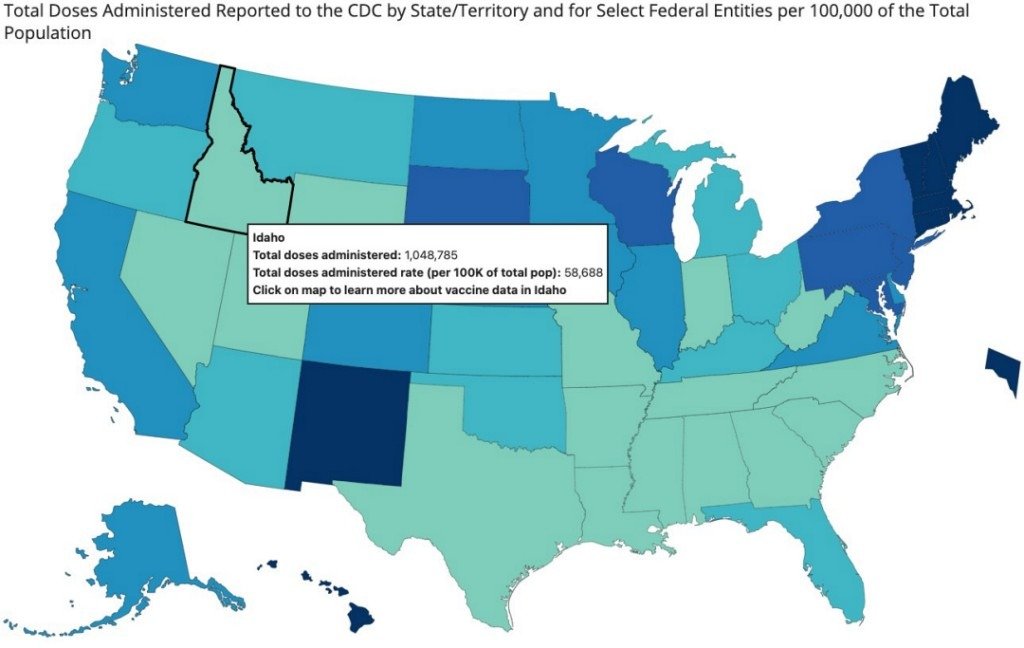
The Centers for Disease Control and Prevention (CDC) reports that so far, just over 40 percent of people in the United States have received at least one dose of a COVID-19 vaccine, and nearly 30 percent are fully vaccinated (as of April 26, 2021). However, the available vaccines are not for kids. So the obvious question is when will there be a COVID-19 vaccine for kids?
Three vaccines have been authorized for adults. One of them can be given to kids that are at least 16 years old. The U.S. Food & Drug Administration has been asked by one vaccine maker to extend its vaccine authorization for kids as young as the age of 12. Clinical trials have even started in kids as young as six months. This brings up several questions that parents are asking as they search for peace of mind about a COVID-19 vaccine for kids. Here are some of the questions parents are asking.
What Should I Know About Clinical Trials For Kids?
The clinical trials must be completed before a vaccine is ready for kids. The reason for the trials is to make sure that any vaccine being tested is safe and effective for young children. When there is enough evidence from the trials, the American Academy of Pediatrics (AAP) will make recommendations for a COVID-19 vaccine for kids.
How Do Trials Work?
It will start with older children. They will be given a range of doses. This allows researchers to find the one that will trigger the strongest immune response without causing too many side effects. After the best dose is known, there will be thousands of participants randomly chosen to receive either two doses of vaccine or of a placebo injection. Those kids will be monitored by researchers for months — at least — as they study the safety and effectiveness of the vaccines. Parents must approve of their child being included in the trials.
When Will A COVID-19 Vaccine For Kids Be Ready?
This all depends on what happens with the clinical trials. If research and progress continue at the pace it is currently going, there is a chance that some kids (the older ones) will be able to get a vaccine before school begins in the fall of 2021. For younger kids, the timeline is looking more like the beginning or middle of 2022 until one is ready. The younger the child, the longer it is expected to take until a vaccine is ready.
Is A Vaccine Necessary For Kids?
Health experts are in agreement that kids will need a vaccine once it becomes available. Consider this quote from Sean O’Leary, MD, vice-chair of the American Academy of Pediatrics Committee on Infectious Diseases and a professor of pediatrics at the University of Colorado Anschutz Medical Campus. “We know kids are not as affected, but it’s inaccurate to say it’s a benign condition in kids. “We need a vaccine for kids to protect them. Younger kids appear to be less likely to get COVID and spread it than adults, but adolescents look more like adults in terms of getting it and spreading it.” So far, 3.34 million kids in the United States have already had COVID-19 and more than 250 of them have died.
Will It Be Required For School?
When there is a vaccine that has been approved for kids, then the leading health authorities like the CDC and AAP will make their recommendations about how it should be handled for children. But when it comes to what happens at school, it will be decided by the government of each state.
Will The Kids Vaccine Be The Same As The Adults?
The vaccines being tested for kids are the same that have been approved for adults. They work by telling cells inside our bodies to make a harmless protein and the body learns to fight the real COVID infection if it shows up. For most vaccines, the dose is basically the same for children and adults. But for a COVID-19 vaccine for kids, that will be known when more research is completed.
What About Side Effects?
This is another reason why clinical trials are so important. As more information is collected, researchers will have a better idea about any possible side effects. To this point, they have no evidence that side effects will be any worse for children.
Which Companies Are Testing A Vaccine For Kids?
Both Pfizer-BioNTech and Moderna have trials underway in children as young as 6 months. The Moderna study started in March. They’ll be testing their vaccine in kids between six months and 12 years old. It will test two or three dose levels and be given in two shots that are 28 days apart. Moderna expects to enroll nearly 7,000 kids in the U.S. and Canada for this trial. They have an additional trial going with 3,000 participants ages 12-17 that compares a dose of the vaccine with a placebo.
Pfizer-BioNTech finished enrollment for their trial in February with 3,000 adolescents. They have recently announced their plans to begin another trial — for kids ages 6 months to 12 years. This trial will begin with the best doses in 144 children. Later, another 4,500 participants will be enrolled in the U.S. and Europe.
Just 4 Kids Urgent Care
We hope that this information helps give you some additional peace of mind when it comes to what is happening with a COVID-19 vaccine for kids. If you have any other questions or concerns about vaccines for your child, wearing a mask, our telemedicine visits, or anything else — give us a call. We are here to help and will take great care of your kids. Don’t hesitate to give us a call!